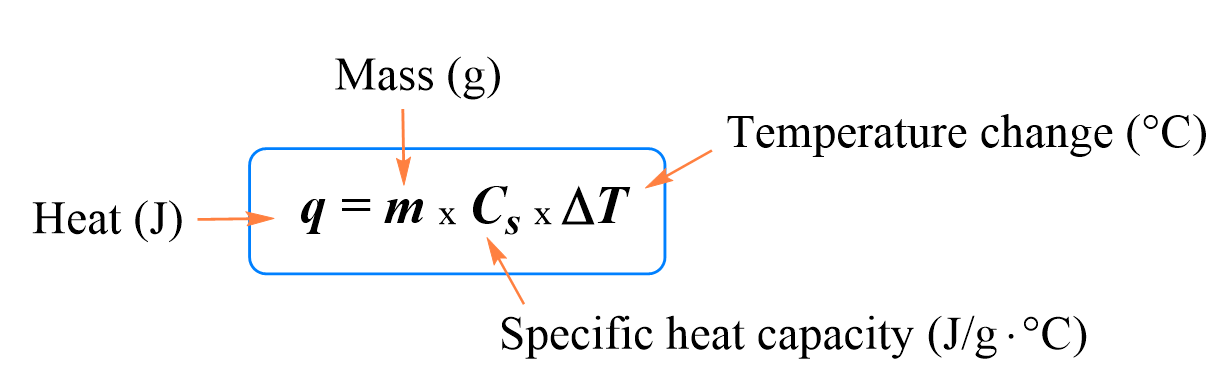
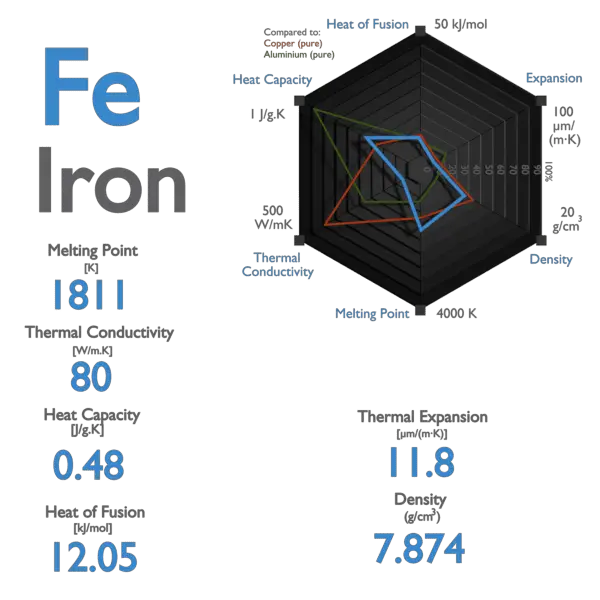
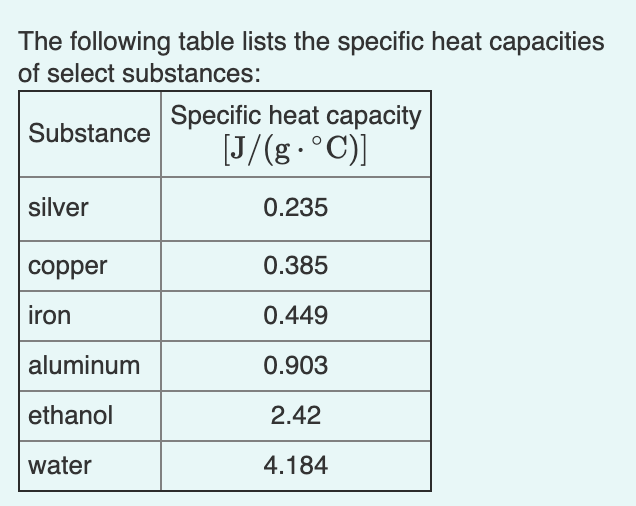
Calculate the amount of heat required to raise the temperature of 5 g of iron from `25^(@)C \"to\" - YouTube

How will you prove experimentally that different substances have different specific heat capacities?
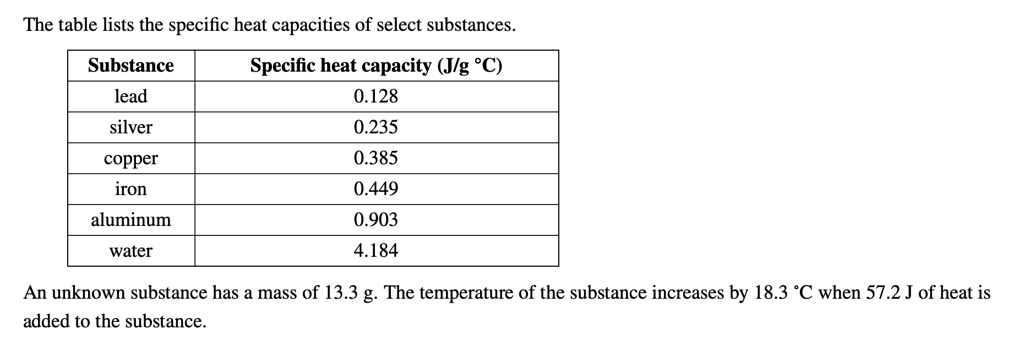
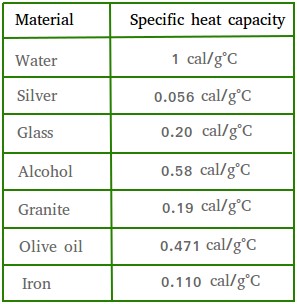
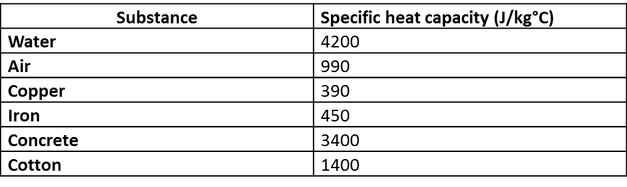
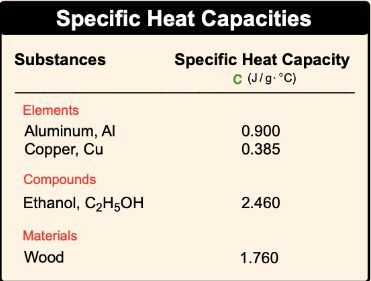
SOLVED: The table lists the specific heat capacities of select substances: Substance Specific heat capacity (J/g°C) lead 0.128 silver 0.235 copper 0.385 iron 0.449 aluminum 0.903 water 4.184 An unknown substance has
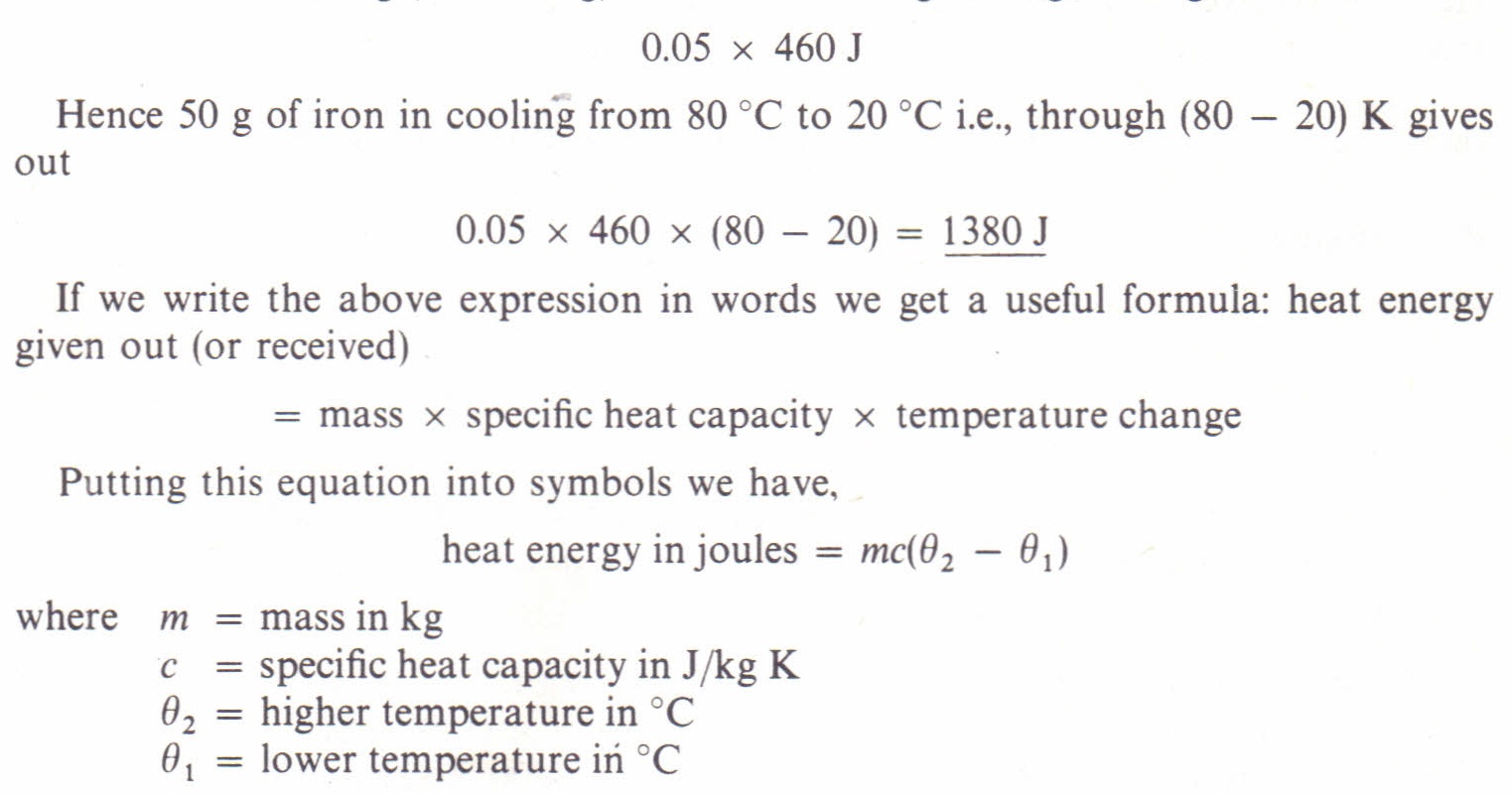
Cheat calculations Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online

A piece of iron of mass 100g is kept inside a furnace for a long time and then put in a calorimeter of water equivalent 10g containing 240g of water at 20^oC .

Color online) Temperature-dependent specific heat capacities of (a)... | Download Scientific Diagram
Why isn't the specific heat capacity of brass the average of the specific heat capacity of its components (which are copper and zinc)? Both copper and zinc have values of 376.8 J/(kg*K)

How Will You Prove Experimentally that Different Substances Have Different Specific Heat Capacities? - Science and Technology 1 | Shaalaa.com